Overview of Drug Discovery and Development
 Drug discovery and development is costly and time consuming process. For a new drug to get approved in US, it might take 10-15 years and cost around 1 billion dollars.
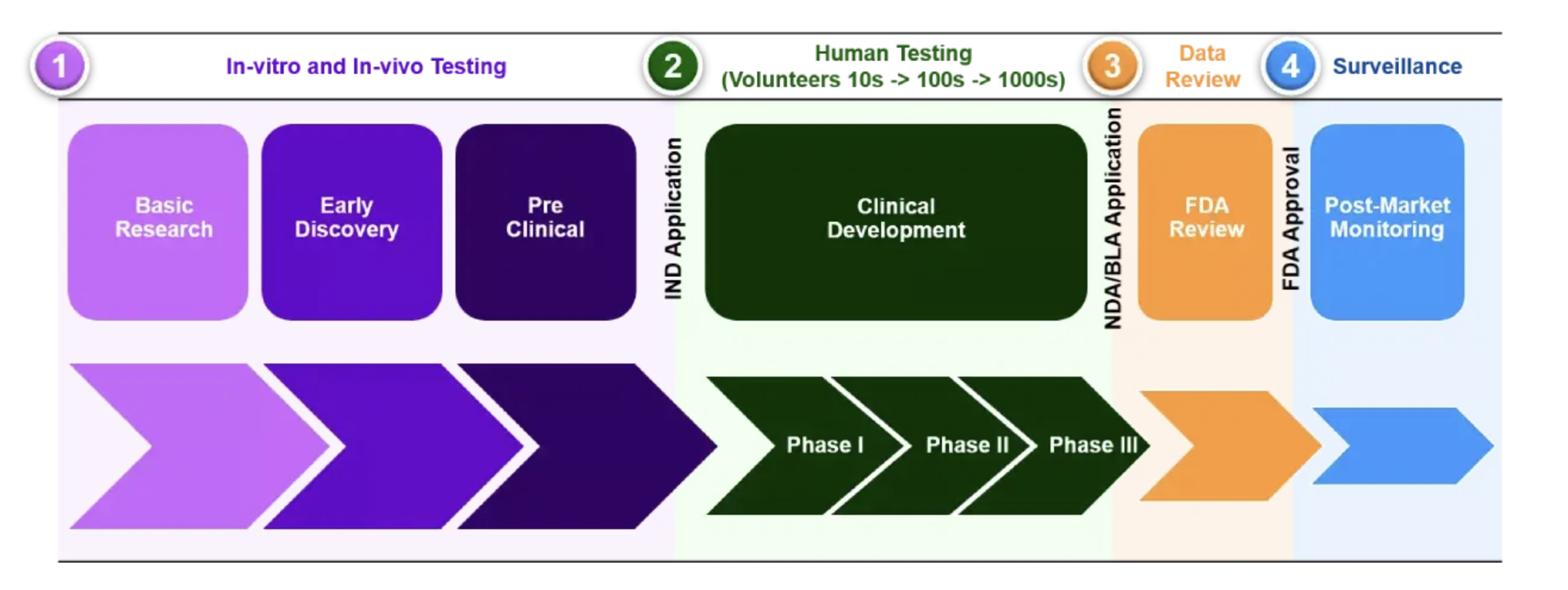
In this post, I go though overview of typical/traditional drug development pathway. These are the phases of drug development, however, each of them could have multiple stages.
Drug discovery and development is costly and time consuming process. For a new drug to get approved in US, it might take 10-15 years and cost around 1 billion dollars.
In this post, I go though overview of typical/traditional drug development pathway. These are the phases of drug development, however, each of them could have multiple stages.
- Early Discovery and Development
- Preclinical Research
- Clinical Development
- Regulatory Approval (FDA in US)
- Post-Market Safety Monitoring
Early Discovery and Development
In this phase, researchers collaborate to identiy and optimize potential leads to a specific target.Essentially, the leads must elicit a desirable effect on a specific biological target implicated in a disase, in the hopes of treating it. Let’s get more in depth in the diffirent processes that occur during the early drug discovery.
Target Identification and Validation
One of the key factors in designing a good drug is having a crystal clear understanding of the pathogenesis of a disease. A suitable biological target is said to be “druggable” when a therapeutic molecule, called a “hit”, can modify its biological activity. Potential targets can may be discovered by sourcing available databases, and researching public literature. Target identification finds a gene or protein that plays a significant role in a disease. Afterwards, scientists and researchers record the target’s therapeutic characteristics. Once target is identified, researchers validate their suitability for drug development. To validate targets, researchers use model tools and techniques such as disease association, bioactive molecules, cell-based models, protein interactions, signalling pathway analysis, antibodies and chemical genomics. For example, the Sanger Whole Genome CRISPER library and Duoling PLA are excellent sources for drug discovery targets.
High Throughput Screening (HTS)
HTS is often one of the most important steps towards discovering a drug. Once a potential target has been identified and validated, the starting point can be detected by screening a large of molecules that can interact with it.
Hit Identification and Discovery
To identify a hit molecule, various methods can be used. The hit is identified as a molecule that must interact with the previously mentioned target to result in a desired therapeutic effect. High Content Screening(HCS), phenotypic screening, fragment-based screening, structure-based screening and virtual screening are examples from large list of strategies used to discover hits.
Assay Development and Screening
Assays are the test systems that evaluate the effects of the new drug candidate (hit) at the cellular, molecular and biochemical levels. Assay development is a crucial component of drug discovery workflow. High Content Screening uses robotics, data processing and sensitive detectors to rapidly conduct millions of pharmacological, chemical and genetic tests, eliminating hours of painstaking testing by scientists. HTS and HCS used to identify active compounds, genes or antibodies that affect human molecules.
Hit-To-Lead (H2L)
As we mentioned earlier, a hit is a term used to describe a chemical compound that has a desired therapeutic effect at a known target molecule. Similarly, the lead is the product of the screening (HTS and HCS) process, which can be used in advanced stages. In other words, a hit is any compound that is confirmed to have binding activity to the targeta and a lead is a hit worth investigating further. A hit might not be worth investigating if it is poorly druggable or toxic or an artifact of the screen or has other problems.
The main aim of the H2L is to find the appropriate leads to move along the pathway to a final clinically active drug.
Lead Generation and Optimization
Once the lead is generated in the H2L process, the lead optimization will commence.During Lead optimization, the lead compounds discovered in H2L process are synthesized and modified to improve potency and reduce side effects. Lead optimization aims to improve the most promising compound products to enhance effectiveness, lower toxicity or increae absorption.
Preclinical Research
Once lead compound is found, preclinical phase of drug development begins with in vivo/vitro research to determine the efficacy and safety of the drug.
In Vivo and In Vitro Assays
-
In vitro (Latin for “in glass”) refers to studies done externally of a living organism. This can include work done on bacteria or mammal cells in a petri dish, for instance
-
In vivo (“within the living”) involves research conducted within the body of a living model. Around 30% of drugs which have passed in vitro pre-clinical studies fail clinical trials. Performing in vivo assays is an essential step in the Drug Discovery process as it reveals drug effects on whole, complex organisms.
These testing arrays are used to test the safety and potential toxic effects of a compound using animal models, alternative models like Zebrafish or cell cultures. Preclinical tests are done on non-human subjects.
In silico Assays
In silico assays are also used in preclinical phase. These are the test systems or biological experiments performed on a computer or via computer simulation. These are expected to become increasingly popular with the ongoing improvements in computational power and bevahoural understanding of molecular dynamics and cell biology.
Clinical Development
Once preclinical research is complete, researcher move on to clinical drug development, including clinical trials and volunteer studies to finetune the drug for human use. Clinical trials must be safe and efficacious and be completed under the drug development budget, using methodology to ensure the drug works as well as possible for its intended purpose. Dose determination also happens in this pahse. Proper dosing determines medication effectiveness, and clinical trial examine dose escalation and multiple dose studies to determine the best patient dosage. Clinical trials happen in three phases.
Phase I
This phase is the first time the drug is tested on humans, less than 100 volunteers will help researchers assess the safety and pharmacokinetics, absorption, metabolic and elimination effects on the body, as well as side effects for safe dosage ranges.
Phase II
Phase II assesses drug safety and efficacy in an additional 100-500 patients, who may receive a placebo or standard drug previously used as treatment. Analysis of optimal dose strength helps create schedules while adverse event and rists are recorded.
Phase III
Phase III enrolls 1000-5000 patients, enabling medication labeling and instructions for proper drug use.
Pharmacodynamic (PD) Biomarkers
PD biomarkers are molecular indicators of the drug’s effects on the target human area, and link drug regimen and biological responses. This data can help select rational combinations of targeted agents and optimize drug regimens and schedules.
Pharmacokinetic (PK) Analysis
During phases, drug dynamics data within the body will be derived and described. PK analysis is the process of analysing this data.
Bioanalytical Method Validation
The validation of bioanalytical methods involves quantative determinations of the corresponding analyte in biological matrices such as urine, saliva, blood etc.
Regulatory Approval
When the development of a drug is almost completed, the approval documentation is drawn up and submitted to the competent authority. The approval authority checks all data and decides on approval. To do this, the drug must have a favorable risk-benefit ratio. The risk-benefit ratio is the most important approval criterion.
Post Market Safety Monitoring
Even after approval has been granted, the risk-benefit ratio should be continously monitored. If it changes, this has an impact on the approval, and in the worst-case scenario, the drug even has to be withdrawn from the market.
In this post, I tried to explain all the phases and their details of the drug discovery and developement. In the future posts, I plan to explain how AI can help to optimize and fasten this process.
Resouces
- https://www.nebiolab.com/drug-discovery-and-development-process/
- https://blog.biobide.com/the-drug-discovery-process