The Immune System: Part III
Note: The Immune System: Part II is prerequisite in order to understand this part better. We will refer to some of the terminologies and concepts defined in the previous part.
The Murder University of the Thymus
The Adaptive Immune Cells have to graduate from: The Murder University of the Thymus. Your Thymus is absolutely crucial for your survival and, in a way, will decide at what age your will die, so you might think that it would be as well-known as liver, lungs or heart, but wierdly enough, most people are not even aware that they have this organ.
Thymus is one of your most important immune cell universities (others include your bone marrow for your B cells, will explain later). Some of your most powerful, crucial adaptive immune cells are educated and trained here: T Cells.
We met one group of T cells on the battlefield before, briefly, when they came rushing in to swing the battle, although we have not even begun to discover all their qualities. T Cells do a variety of things, from orchestrating other immune cells, to being antivirus superweapons, to killing cancer cells. Without T Cells you are quite dead – they may be the most important Adaptive Immune Cell you have. But before they can fight for you, they need to pass the horribly dangerous curriculum in the Thymus. Failing a test here means death.
The Adaptive immune system is mixing gene segments to produce an amazing variety of diffirent receptors, able to connect to every possible protein, in this context called antigens, in the unvierse. This means that each individual T Cell is born with ONE specific type of receptor, able to recognize ONE specific antigen. But there is a vital flow: With so many diffirent receptors there are guaranteed to be a large number of T cells with receptors that are able to connect to proteins from your OWN cells. This is not a theoretical danger, but the cause of a number of very real and serious diseases that millions of people are suffering from right now called autoimmune diseases.
For example, lets say a T Cell receptor can connect to a protein on the surface of a skin cell, it would not understand that it is connecting to a friend. It would just try to kill it. Or worse, since there are quite a lot of skin cells in the human body, it would think a large attack was going on with enemies everywhere, and alert the rest of the immune system to go into attack mode, and cause inflammation and all sorts of chaos.
As you can imagine, the body takes this issue extemely seriously and came up with the Murder University of the Thymus to address it. After a fresh and young T cell has been born, it travels to the university and begins its training which consists of three steps, or better three tests:
The first test is basically just making sure the T Cells have the ability to make working T Cell receptors (recetor is functional or not).\
The T Cells that pass test one have functional receptors. Great job so far! The second test is called positive selection: Here the teacher cells check if the T cells are really good at recognizing the receptors of the cells they will need to work with. Imagine this part as if the teacher is checking if the pens the students brought are full of ink. Once again, death is the punishment for failing second test.
After two tests, the third test is Negative selection. The final exam is simply: Can the T cell recognize self?. Can its receptor connect to the main proteins inside the body? The only acceptable answer is “No, not at all”.
So in the final exam the T cells are presented with all sorts of protein combinations that are used by the cells of your body. The way this happens is pretty fascinating by the way – the teach cells in the Thymus that do the testing have a special license to make all sorts of special proteins that usually are made only in organs like heart, pancreas, or the liver and also hormones, like insulin for example. This way they can show the T cell all kinds of proteins that are marked as “self”. If a T Cell is able to recognize any of these self-proteins, then they are taken out back and shot in the head immediately.
Unfortunately, yor Murder University is already in the process of shutting down. Your Thymus basically begins shrinking and withering away when you are a small child. Every year more Thymus cells turn into fat cells or just worthless tissue. The university closes more and more departments and gets worse as you age, until around the ripe age of eighty five, your T Cell uiversity closes its gates for good.
Antigen Presentation
When an infection occurs, your immune system determines which specific defense is needed and how much of it. The adaptive immune system works with Innate immune system to find the few cells that have the right receptors for this specific invasion, locate them among billions of others in your body, and then rapidly produce more of these cells. How does it do this? By preparing a presentation.
Your Adaptive immune system does not make any real decisions about who to fight and when it is time to activate – this is the Innate immune system’s job, and this is where the Dentritic Cell comes into play. When an infection happens it covers itself with a selection of enemy’s antigens and tries to find a Helper T cell that is able to recognize one of the antigens with its specific receptors. And this is exactly the reason why the Dentritic cell is so crucially important. Without Dentritic cells, there would be no second line of defense.
The first few hours of an infection, the Dentritic cell samples the battlefield and collects information about the enemy, which is a nice way of saying that it swallows enemies and disassemblies them into their parts, or antigens. The Dentritic Cell is an antigen-presenting cell.
The Dentritic cell then travels through the lymphatic system to present them to the Adaptive Immune System, or more precisely, to Helper T Cells.
An antigen-presenting cells have one more thing in common: A very special molecule that is as important Toll-Like Receptors, called Major Histocompatibility Comlpex Class II. Or in short, MHC class II. You can imagine the MHC class II receptor as a hot dog bun that can be filled with a tasty wiener. The wiener in this metaphor is the antigen.
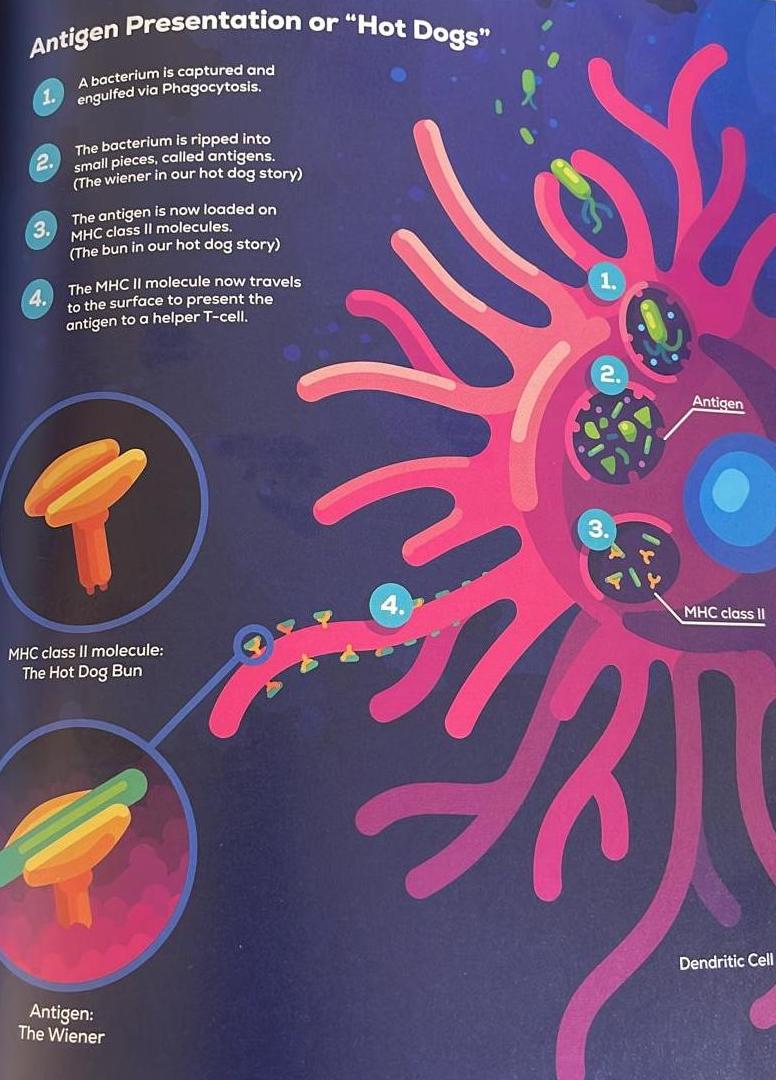
Helper T cells are able to recognize an antigen only if it is presented in an MHC class II molecule. This makes sure that Helper T Cells can not just get activated by accident because they pick up antigens that float around freely i the blood or lymph. They need to be presented with an antigen that sits in an MHC class II, from an antigen-presenting cell.
So back to the battlefield, where soldiers are engaged in an epoc battle, Dentritic Cells awallow a cross section of everything floating around, including enemies. If they grab a bacterium they rip it into small pieces, antigens (wieners), and put them into MHC class II molecules (hot dog buns) that cover its outsides. Then the Dentritic Cell makes its way through the lymphatic system to the closest lymph node to look for a Helper T Cell. When a Helper T Cell happens to have the right T cell receptor, with just the right shape that recognizes the antigen in the MHC class II molecule, it will connect to it. This is pretty exciting moment, the Dentritic Cell atcually has found the right Helper T Cell out of billions. But it still NOT enough to activate Helper T Cell, a second signal is necessary, communicated by another set of receptors on both cells.
This second signal is like a gentle kiss from the Dentritic Cell. It is another confirmation signal that clearly communicates again: “This is real, you are really properly activated!”. Why is that so important tat we mention it here? This is another security mechanism that prevents Helper T Cells from activating accidentally – only if a Dentritic Cell, that is representing Innate Immune System here, is activated by real danger is the Adaptive Immune System, represented by the Helper T Cell, supposed to activate.
Dentritic Cells are snapshots of a battlefield in a moment in time. Once one leaves, it stops sampling and is locked in. After arriving in a Lymph Node, the Dentritic Cell has about a week to find a T Cell to activate before an internal timer runs out and it kills itself, as so many immune cells do. Basically, by sending fresh snapshots or newspapers, every few hours and eventually deleting them, your immune system collects and delivers a constant stream of fresh information about the battlefield. By deleting it regularly it makes sure not to operate on old information.
Awakening the Adaptive Immune System: T Cells
In this section, we will talk about T Cells classes and their details.
T Cells have a much more varied set of jobs than the Macrophages or Neutrophils we got to know earlier. There are multiple classes of T Cells: Helper T Cells, Killer T Cells, and Regulatory T Cells each able to specialize even more into various subclasses, for every possible kind of infection.
T Cells are travellers that start their lives out in the bone marrow, where they mix and match the gene fragment that create their unique T Cell receptors, before they visit the Murder University of the Thymus to be educated. If the T Cell survive their education they move through your lymphatic megacity network, looking for an antigen that is exactly right and to get the encougaring kiss from a Dentritic Cell, to get activated.\
T Cells travel the whole of your lymphatic system once per day, so the chances of meeting exactly the right Dentritic Cell with matching antigen to the T cell receptor works out just fine. When this meeting happens the T Cell activates, and the hell breaks loose.
For now we will just talk about the Helper T Cell to keep things simple, but we will get to know the classes of T Cells much more later on. We talked about the Helper T Cells already but now we are going to get a fuller picture.
The Helper T Cell can not stay alone if it wants to help beat back the infection, so its first job is to make more of itself. What we will pretty casually describe in the next two chapters is called the Clonal Selection Theory. The theory is basically goes like this:
Your activated T Cell leaves the Dentritic Cell that activated it behind and wanders to a diffirent part of the Lymph Node where it begins the process of cloning itself. It divided over and over again, multiplying as fast as it can. Withing hours there are thousands of them. Because each of the clones has the same unique T Cell receptor like the first Helper T Cell that got activated, your immune system now has thousands of cells with this unique receptor.
Once enough clones have been made, the individual cells split into two groups: Let us follow the first group now, we will come back to the second one later! They need a moment to orient themselves and take a deep sniff of cytokines and danger signals that have been carried by the lymph to the lymph node, and then they follow the chemical track to the battlefield as quickly as they can.
Around five days to a week after the wound was inflicted Helper T Cells arrive at the site of infection. They act as commanders and play the role of amplifiers. Helper T Cells do not just put the Macrophages into Killer mode, and keep them alive. Helper T Cells can reset Macrophages suicide timer over and over again, as long as danger is present, they will tell your exhausted warriors to carry on by restimulating them over and over again.
When the battle won, the last thing most Helper T Cells do at the battlefield is to kill themselves, except a few dont. Some Helper T Cells become Memory Helper T Cells. Whenever you hear that you are immune to a disease, this is what this means. It means that you have living memory cells that remember a specific enemy. And that enemy might come back, so they stick around and become powerful guardians. Memory Cells are able to recognize a familiar enemy much quicker than the Innate Immune System ever could. In case of another infection, this maes the long trip of the Dentritic Cell to the lymph node unnecessary because these Memory Helper T Cells can immediately activate and call for heavy reinforcements.
Remember we followed only one group from the lymph node to the battlefield. There was a second group that remained and what they are about to do might be even more important: Activating some of your most immune weapons. The mighty B Cells, your living weapon factories.
B Cells and Antibodies
B Cells are large, blob like fellows that share a few characteristics and properties with T Cells, namely that they originate in the bone marrow and that they have to undergo the same brutal and deadly education– only it does not happen in the Thymus but directly in the bone marrow.
Just like the their T Cell buddies, all your B Cells combined come with at least hundreds of millions to billions of diffirent receptors for millions of diffirent antigens. And just like T Cells, every single individual B Cell has one specific receptor that is able to recognize on spefici antigen.
What makes B Cells special, and very dangerous for friends and foes, is that they produce the most potent and specialized weapon the immune system has at its disposal: Antibodies. Antibodies are weird things and pretty complex and fascinating, so we will discuss them in detail later, but for now, in a nutshell – Antibodies are basically B Cell receptors. Antibodies themselves are a bit like crab-like sniper rifles, as they have been made against a specific antigens and therefore a specific enemy.\
But wait, how can something be a receptor and a weapon that floats around at the same time? So basically, antibodies are stuck to the surface of B Cells and work as their B Cell receptors, which means that they can stick to an antigen and activate the cell. Once a B Cell is activated it begins to produce thousand of new Antibodies and starts vomitting them out, so they can attack enemies – up to 2000 per second. All antibodies are produced this way. Lets focus on B Cells for now, we will talk Antibodies in detail in the next section.
So as we said in the beginning B Cells are born in your bone marrow where they mix and recombine the gene segments responsible for their B Cell receptors to be able to connect to one specific antigen. After they did that, similarly to T Cells, they have to undergo a harsh and deadly education to make sure that they are not able to connect to the proteins and molecules of your body. The survivors become travelling Virgin B Cells, inactive cells that move through your lymphatic system every day, just like T Cells. But this is where the similarities between T and B Cells end.
In the lymph nodes there are specific B Cell areas where they hang out for a bit and have a coffe, waiting a bit to see if they are needed. B Cells are very dangerous cells, so they need a strict two-factor authentication to be truly activated–one by Innate Immune System and another by Adaptive Immune System. Now, lets go through each step in detail
- Step one: B Cell activation by the Innate Immune System:
We did learn before what your body does with excess fluids in your tissue, it consistently washes them away, right into the Lymphatic System. The fluid and with it a lot of the battlefield detritus, with parts of dead bacteria and spent cytokines and other garbage, become part of your Lymph. This way the lymph flowing through you is a liquid information carrier. And this information is headed towards the next immune system base, the megacities and intelligence centers of the lymph nodes. Once it arrives here it is drained through the B Cell area where thousands of virgin B Cells hang out. The B Cells get right in the middle of the stream of fluid information and let the lymph flow around them and their B Cell receptors, which sift and explore all the antigens and detritus coming from the tissue.
The virgin B Cells look specifically for antigens they can connect to with their special and unique B Cell receptors. They are fishing for the one antigen then can connect to, so they know they can activate!
So far so good, but you may have noticed something: There is no Dentritic Cell involved here, so does this mean B cells dont need to go through this dance with another cell?? It all has to do with a huge difference between T Cell receptors and B Cell receptors.
Remember the MHC class II molecule? The hot dog bun that presented an antigen, the wiener, to the T Cell receptors, so it could activate? T Cell receptors are really picky eaters that eat only weiener and only if they come inside a bun. But this has major consequence for T Cells: The antigens that can activate T Cells have to be short because the MHC molecule can only carry short antigens. In contrast, the B Cell receptors are not as picky.
Both T and B Cell receptors are each made to recognize a specific antigens, but your B Cells are far less restricted. So T and B cells recognize very diffirent things/antigens in size and dimension. B Cells can pick antigens right from the fluids around them and activate. B Cells dont need and MHC molecule, they dont need to get a presentation from another cell like T cells do.
There is more: B Cells do have more direct help from Innate Immune System: B Cells can not just recognize the dead bacteria antigens, they also have special receptors that are able to recognize complement proteins.
We mentioned before that the Innate Immune System is responsible for activating and providing context to the Adaptive Immune System and here we are encountering this principle once more! By being attached to the pathogens, the complement system is officially confirming to the B Cells that there is a real danger. So complement proteins attached to an antigen makes it about 100 times easier to activate a B Cell than it would be to activate it without the complement.
OK, so what does this early activation look like? Well, first of all the activated B cell moves to another area in the lymph node and begins cloning itself. This cloning continues until there are around 20,000 identical clones, all with copies of the specific receptor that was able to connect to the original antigen, the first virgin B Cell picked up. These B Cell clones begin producing Antibodies that use the blood as a lift to the site of infection and can flood the battlefield and help out.
Without second step, without activation number two, most of these B Cell clones will kill themselves within a day. Which makes sense actually, because if they dont get activated again, these B Cells have to assume the infection was pretty mild and they are not actually needed that badly, so not to waste any resources, they kill themselves.
To be truly awakend we need the second part of the two-factor authentication. And this one is provided to the B Cells by their collegues from the Adaptive Immune System, more precisely, by activate Helper T Cells.
- Step two: B Cell Activation by the Adaptive Immune System:
As we learned in the last chapter, after a Helper T Cell has been activated and has made a lot of clones of itself, one group of the Helper T Cells moves to the battlefield while the other group goes off to activate B Cell for real.
In a nutshell, an activated T cell need to find an activate B Cell and BOTH cells need to be able to recognize the same antigen! Ok, wait a second, so are we seriously saying that two cells in your body mix gene fragments randomly, with hundreds of millions of possible outcomes? And then a pathogen shows up and coincidently both need to be activated independently and then they need to meet each other? And only then, in this specific and seemingly impossibly unlikely case will your immune response fully be activated? Well, YES!
Basically, these are the steps happens:
- A battle needs to occur and dead enemies, which are big chunks of antigens, need to float through the lymph node. Here, a B Cell, with a specific receptor needs connect to the antigen. If the dead enemy is covered in complement, activation will be much easier. This will activate the B cell, which makes a lot of copies of itself, and produces low-grade antibodies, but the B Cells will die after around a day if nothing more happens
- In the meantime, a Dentritic Cell needs to pick up enemies at the battlefield and turn them into antigens which are put in the MHC class II molecules and travel to the T Cell dating area in the lymph node. Here it needs to find a Helper T Cell that is able to recognize the antigen with its unique T Cell receptors. If it happens the Helper T Cell is activated and makes a lot of copies of itself.
- The B Cell breaks the big chunk of antigen down into dozens or hundred of small antigens and begins presenting them in MHC class II molecules
- An activated B Cell that is presenting hundred of diffirent antigens needs to meet a T Cell that can recognize one of these antigens with its specific T Cell receptor, which is the second signal for the B Cell.
Only if this exact sequence of events occurs does a B Cell get activated for real. THIS IS IMPRESSIVE! Once more: THIS IS MIND-BLOWING!
Your B cell that was properly activated through the two-factor authentication now changes. It begins to swell, to almost double its size, and transforms into its final form: Plasma Cells.
The Plasma Cell now begins producing antibodies for real. It can release up to 2000 antibodies per second that saturate the lymph and blood and the fluids between the tissue.
Antibodies
Antibodies are among the best and most specialized weapons your immune system has at its disposal. Produced by B cells, antibodies themselves are not particularly deadly. They are actually nothing more than mindless protein bundles that can stick to antigens. But this they do with extreme efficiency.
You can imagine them as a sort of hashtag of death. The most common antibodies are shaped like little crabs with two pincers and they are seriously pretty tiny. In a sense they are sort of comparable to the proteins of the complement system – which are also nothing more than tiny proteins that float around – but with one big difference: Complement proteins are generalists, while Antibodies are specific.
Antibodies are extremely good at grabbing on to the enemies they are made for with their two pincers. And they have cute little butts that are extremely good at connecting to your immune cells. The pincers are for enemies, the cure butts are for friends.
With these tools Antibodies do multiple things: First, similar to complement, they can opsonize(make foreign cell suspectible to phagocytosis) enemies. In this context, it means that Antibodies swarm and enemy and grab them, which makes their victim more delicious for your soldier cells to eat.
When an antibody army arrived at our infected toe, the bacteria that were covered by them were equally unhappy with thier life situation and they are helpless. But antibodies dont only make pathogens helpless, they also can maim/injure them and make them unable to move. Or in the case of viruses antibodies can directly neutralize them and make them unable to infect cells.
Here another safety layer of your immune system comes in. The cute butts of antibodies that are for friends are in a sort of “hidden mode” when antibodies just float around, so immune cells can not just pick them up from fluids. As soon as an antibody has grabbed a victim with its tiny pincers, its butt changes its shape and is now able to bind to immune cells. This is pretty important since your body is teeming with antibodies at any time, and it would cause all sort of chaos if your immune cells bind to the antibody-butt just randomly.
Another thing antibodies can do with their cute butts is to activate the complement system. Remember, complement is just kind of floats passively in the lymph. And some bacteria are able to hide themselves from he complement system so that it does not activate close to them. Antibodies are able to activate the complement system and sort of attract it to the bacteria, increasing its efficiency wildly. Again we see the principle of our two immune systems: The innate part does the actual figthing, but the adaptive part makes it more efficient with deadly precision.
Antibodies are not just tiny crabs though. There are multiple classes that actually do very diffirent things and are used for diffirent situations. I will not expain them in detail, however will mention their names for curious readers.
- IgM Antibodies: The first Defenders on Site
- IgG Antibodies: The specialists
- IgA Antibodies: Making poop and Protecting Babies
- IgE Antibodies: Allergy haters
Conclusion
This is the end of my series of posts about immune system. First of all, thank you very much for reading them. Much Appreciated! I tried to summarize what I learned from the book authored by Philipp Dettmer called “Immune”. The book covers much more details with beautiful pictures. It is an amazing book, highly recommended.
Secondly, I learned a ton about human immune system, and this made me much more curious and interested in further details. Planning to write more about the related topics in the future.